Adoption of Novel Diagnostic Testing in Prosthetic Joint Infection
Lead: Madeline Warren
Recent advances in diagnostic testing for prosthetic joint infection have led to an expansion of options available for use in the clinical setting. Each has different sensitivity and specificites. Availability and application of novel diagnostics vary significantly between hospitals. Our lastest study aims to reveal current clinical practice within the region and how extensively novel diagnostics are used.
If you would like to take part, please register via email with name, grade and hospital trust and a named supervising consultant: Orcapaedics+PJI@gmail.com
The contributor and supervising consultant from each Trust will be listed as collaborative authors on any future publication from this study.
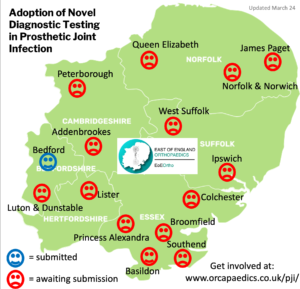
Please note that only one response can be submitted per hospital and the first collaborator to sign up will be registered. We have already received responses from some Trusts- see below:
Our Frequently Asked Questions (FAQ) page includes information about authorship etc.
Got a research idea?
Then share your thoughts with us and we will try to help you put it into action throughout the region.